PharmaNest provides high-resolution, single-fiber phenotypic quantification of fibrosis from digital images of conventional stained slides
for clinical and pre-clinical studies
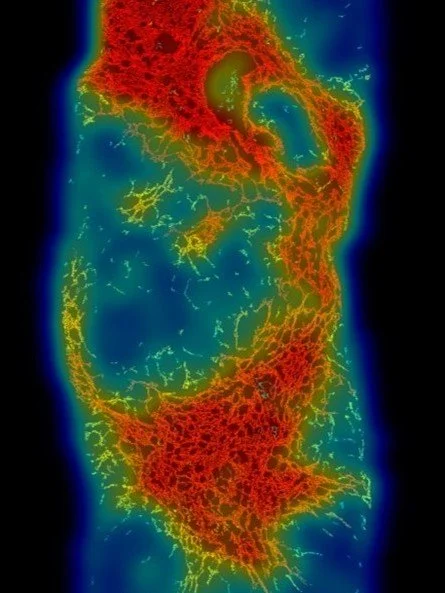
FibroNest analysis of a liver biopsy of a patient with MASH
Why Single-Fiber, High-Resolution Analysis Matters?
Fibrosis is not a uniform process—it’s a complex reorganization of collagen fibers that reflects the balance between injury and repair. Traditional histological scoring and bulk collagen measurements often overlook the micro-architectural details that drive disease progression or regression.
Single-fiber, high-resolution analysis captures these nuances. By quantifying each collagen fiber’s geometry, thickness, orientation, and connectivity, it reveals patterns of tissue remodeling that correspond to biological mechanisms and treatment effects. This approach enables:
Sensitivity: Detects subtle, early changes before they are visible by conventional scoring.
Objectivity: Provides continuous, reproducible measurements independent of reader bias.
Spatial Insight: Maps fibrotic micro-environments across tissue layers and regions.
Translational Power: Links preclinical fiber phenotypes to clinical outcomes and therapeutic response.
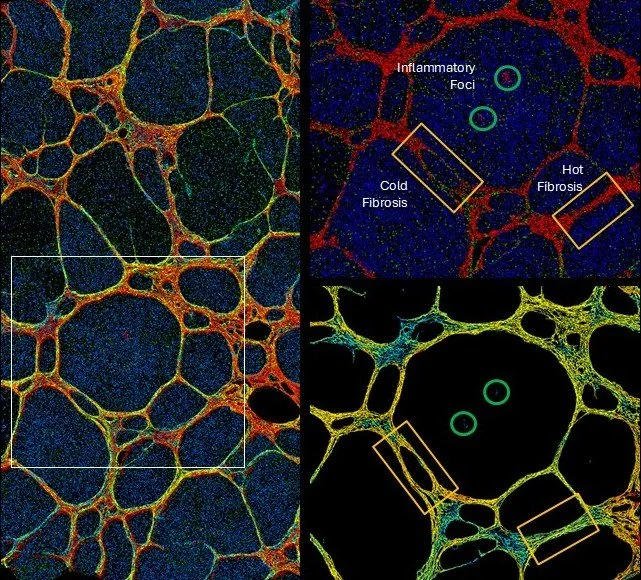
Fibrosis in Its Inflammatory and Immune Context
Fibrosis does not occur in isolation. Each collagen network carries the imprint of the inflammatory environment in which it forms.
By quantifying fibrosis together with its local inflammatory context (from H&E or fluorescence images for instance), PharmaNest’s digital pathology platform reveals biological patterns that are invisible to conventional histology - and essential for drug development to
Distinguish active from chronic remodeling
Capture spatial treatment effects
Clarify mechanisms of action
Strengthen biomarker performance
Fusion of Single Nuclie and Single Fiber Analysis
Seamless Integration into Conventional Digital Pathology Workflows.
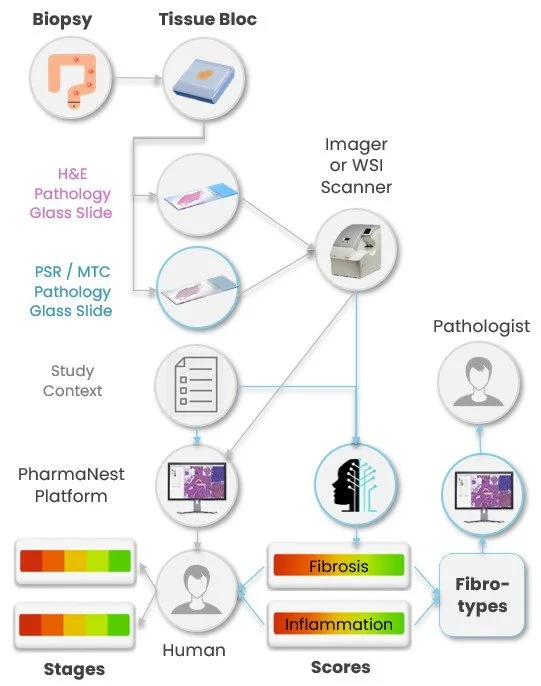
The PharmaNest™ digital Pathology platform is designed to integrate into existing digital pathology workflows, minimizing disruption while maximizing analytical value. It operates directly on standard whole-slide images (WSI), without requiring additional staining, scanning, or image preparation.
Through its interoperable architecture, the platform connects and accepts common Digital Images slide formats and histological stains, as well as any associated anonymized numerical or textual information enhancing the contextual interpretation of a study.
Scores and Fibrotypes are generated by FibroNest and FibroMAP alone or in conjunction with study context data or other Spatially resolved datasets
PharmaNest services are delivered worldwide via the cloud
Key Benefits of Seamless Integration:
No Workflow Disruption: Uses standard Digital Pathology slides, no new protocols needed.
Contextual Interpretation: integrates with digital clinical or pre-clinical study contextes
Rapid Deployment: PharmaNest services and platform are deployed worldwide via the cloud.
Traceability & Compliance: All analyses are audit-trailed and align with regulatory standards for clinical and preclinical studies.
Enhanced Collaboration: Results can be shared across pathology teams and biostatistics groups in real time.
Scalable Data Generation: Converts entire slide archives into structured, quantitative fibrosis datasets
In essence, PharmaNest’s services extends the power of traditional digital pathology—turning histology slides into quantitative, reproducible, contextual and translationally relevant fibrosis and inflammation data without changing how pathologists already work.
Full Translational Capabilities
Using a consistent analytical framework, FibroNest™ bridges preclinical discovery and clinical translation through quantitative, single-fiber analysis and spatial profiling of fibrosis across species and therapeutic modalities.
With its companion module FibroMAP™, FibroNest identifies reproducible spatial phenotypes, or fibrotypes, that capture distinct tissue remodeling patterns linked to disease stage or treatment response.
PharmaNest provides a unified translational bridge connecting digital histology, biology, and clinical outcomes.
Key Translational Strengths:
Cross-Species Continuity: Uses a consistent analytical method from preclinical to clinical settings
Mechanistic Insight: Identifies distinct fibrosis phenotypes and microarchitectural changes that correspond to drug response.
Regulatory Alignment: quantitative, auditable metrics are suitable for inclusion in biomarker qualification, IND submissions, and endpoint harmonization.
Therapeutic Breadth: Deployed across multiple disease areas to translate biological insights.
Predictive Power: Links histological fibrotypes to functional and clinical outcomes.